Home
/ Nutriënten / ...
Samenstelling visolie
Visolie
wordt doorgaans gewonnen uit makreel, haring,
tonijn, zalm,
sardines en ansjovis en
ontdaan van vervuilingen zoals zware metalen.
In
visolie zitten zowel verzadigde vetten als meervoudig onverzadigde vetzuren
(vooral omega-3 en omega-6), doch ook enkelvoudig onverzadigde vetzuren
(omega-9).
Vissen
rijk aan omega-3 vetzuren maken die omega-3 vetzuren niet zelf aan doch krijgen
die binnen met hun voeding. Hun belangrijkste voeding is krill.
Krill zijn garnaalachtige kleine ongewervelden die behoren tot de
orde Euphausiacea. Krill
voedt zich met plankton en microalgen, waarin omega-3 vetzuren ook in de vorm van ALA
zitten.
Krill zet ALA om in EPA en DHA, en
wel in de vorm van fosfolipiden. Vissen
eten krill en worden op die manier rijk aan EPA en DHA, maar krill wordt ook
gebruikt om krillolie te maken.
Visolie
gemaakt van microalgen blijkt volgens onderzoek
wat minder opgenomen te worden in het lichaam dan visolie gemaakt van vissen.
Er
is een groot verschil in kwaliteit van visolie die te koop is.
Goede
visolie bevat de natuurlijke vetzuren, trilgyceriden (TG) en/of
fosfolipiden zoals die in vis
zitten.
De
meest verkochte visolie bevat echter veresterde vetzuren (EE). Door
Transesterificatie (of omestering genoemd), waarbij de glycerolen in de
triglyceriden vervangen worden door ethanol (alcohol) ontstaan uit natuurlijke
visolie de ethyl esters. Dan wordt door moleculaire distillatie de hoeveelheden
EPA en DHA vergroot. De nu verkregen visolie, bevat alleen ethyl esters en zeker
geen vetzuren en wordt daarom ten onrechte visolie of geconcentreerde visolie (Fish
oil concentrate) genoemd.
Uit
verschillende onderzoeken (zie beneden) blijkt dat de EE aanzienlijk slechter
opgenomen in het lichaam dan de TG.
Er
is ook al onderzoek dat laat zien dat EE niets
doet voor een betere bloeddruk in tegenstelling tot TG.
Een
ander groot nadeel van EE is dat deze vetzuren ook veel sneller kunnen oxideren
bij blootstelling aan licht, warmte of zuurstof dan de triglyceriden.
Volgens
onderzoek zijn de meeste van deze
producten die verkocht worden al geoxideerd en kunnen de voordelen van inname
hiervan wel eens nadelen worden.
Als
niets op de verpakking van het supplement vermeld staat weet u bijna zeker dat
het EE betreft, triglyceriden of fosfolipiden zal zeker vermeld staan.
Hoe
u zelf kunt controleren of het EE of TG zijn? Doe wat visolie in een polystyreen
(piepschuim) potje. Als het EE's zijn ziet u binnen 10 minuten dat al redelijk
wat van de olie door het potje heen lekt. Bij TG's of fosfolipiden gebeurt dat niet, hooguit
minieme lekkage na 2-3 uur.
Inmiddels
bestaat er ook een milieuvriendelijker algenolie, rijk aan omega-3 vetzuren,
waarbij geen vissen gebruikt worden. Doch nergens is de juiste samenstelling van
algenolie te vinden m.b.t. de omega-3 vetzuren die er in zitten. Gelet op het
productieproces zullen dat dan bijna zeker ethyl esters zijn.
Fish Oil Triglycerides vs. Ethyl
Esters: A comparative review of absorption, stability and safety concerns
Media exposure, scientific
findings, and word of mouth have lead to a significant increase in fish oil
supplementation over the past five years. The popularity of these supplements
has also lead to an increased concern over product quality. The term
“pharmaceutical quality” is typically associated with fish oils that are
highly refined; however, the use of this term is not regulated and can be
freely used by any branded fish oil product. Most experts associate
pharmaceutical quality with products that comply with a fish oil monograph
developed by the Council for Responsible Nutrition (CRN). The CRN Monograph
established strict limits for environmental contaminants and oxidative quality
parameters. Although the CRN Monograph represents an important step forward in
the standardization of high quality fish oil it does not address every issue
relating to quality. In particular the monograph does not differentiate
between lipid classes (molecular forms). Although a product label may say
“Fish Oil”, the chances are the product is not an oil at all, rather it is
an alternate lipid class called a fatty acid ethyl ester (FAEE) or just EE for
short. This differs in molecular structure from authentic fish oil which has a
chemical structure known as a triglyceride (TG).
What are triglycerides?
The National Academy of Sciences defines fats and oils as “complex organic
molecules that are formed by combining three fatty acids with one molecule of
glycerol”. Triglycerides, or triacylglycerols, are the terms used to define
this molecular structure combining three fatty acids (i.e. EPA and DHA)
esterified (bonded) to a glycerol backbone. TGs are the natural molecular form
that make up virtually all fats and oils in both animal and plants species.
The omega-3 fats present in fish are almost exclusively TGs1.
Because free fatty acids are rapidly oxidized the TG structure offers greater
stability to the fatty acids and prevents breakdown and oxidation2.
What are ethyl esters, and how are they produced?
Fatty acid ethyl esters are a class of lipids that are derived by reacting
free fatty acids with ethanol (alcohol)3. Called
trans-esterification, the process involves a reaction whereby the glycerol
backbone of a TG is removed and substituted with ethanol4. The
resulting EE allow for the fractional distillation (concentration) of the long
chain fatty acids at lower temperatures. Commonly referred to as molecular
distillation in the fish oil industry this step allows for the selective
concentration of the EPA and DHA fatty acids to levels greater than found
naturally in fish3. The resulting EPA and DHA concentrate is typically the end
product that is subsequently marketed and sold as “Fish Oil concentrate”.
This situation presents several issues. Because the term fat or oil refers
only to TG the EPA and DHA ethyl ester concentrate is, by definition, no
longer a fat or oil and is incorrectly marketed as fish oil. Because EEs
rarely occur in nature this affects the way they are digested and absorbed in
the body.
Are all fish oil concentrates ethyl esters?
The vast majority of fish oil concentrates sold globally; including those sold
in North America are EPA and DHA EE concentrates. A small percentage of fish
oil concentrates on the market are natural TGs. In the manufacturing of EE
concentrates it is possible to convert the fatty acids back to TGs using food
grade enzymes. This process, called glycerolysis, removes the ethanol molecule
and re-esterifies the EPA and DHA fatty acids to a glycerol backbone. These
are commonly referred to as re-esterfied or concentrated triglycerides (rTGs).
The process of converting EE to TG is uncommon due to cost constraints adding
30-40 % to the end bulk oil cost. Therefore, the only rationale for omitting
the glycerolysis step is cost cutting.
Absorption and metabolism of natural triglycerides vs. ethyl esters
Dietary fish oil (triglycerides) is digested in the small intestine by the
emulsifying action of bile salts and the hydrolytic activity of pancreatic
lipase1,5. The hydrolysis of a TG molecule produces two free fatty
acids (FFA) and a monoglyceride (one fatty acid combined to glycerol)1,5.
These metabolic products are then absorbed by intestinal enterocytes and
reassembled again as TGs1,5. Carrier molecules called chylomicrons
then transport the TGs into the lymphatic channel and finally into the blood6.
The digestion of EEs is slightly different due to the lack of a glycerol
backbone1. In the small intestine it is again the pancreatic lipase
that hydrolyzes the fatty acids from the ethanol backbone, however; the fatty
acid-ethanol bond is up to 50 times more resistant to pancreatic lipase as
compared to hydrolysis of TGs7,8. The EEs that get hydrolyzed
produce FFA plus ethanol. The FFA’s are taken up by the enterocytes and must
be reconverted to TGs to be transported in the blood1. The TG form
of fish oil contains its own monoglyceride substrate; whereas EE fish oils,
coupled to ethanol, do not. EE must therefore obtain a glycerol substrate from
another source. Without a glycerol or monoglyceride substrate TG re-synthesis
is delayed, suggesting that transport to the blood is more efficient in
natural TG fish oils in comparison to EEs. Furthermore, this delay of TG
re-synthesis in EE fish oils could cause an increase in free fatty acids and
subsequent oxidation of those free fatty acids.
Bioavailability of triglycerides vs. ethyl ester fish oils
Numerous studies have assessed the absorption and bioavailability of EE fish
oils. Most studies have measured the amount of EPA and DHA in blood plasma
after ingestion of fatty acids as either TG or EE. Although a few studies have
found that the absorption rate is similar between the two types of oils, the
overall evidence suggests that TG fish oils are better absorbed in comparison
to EE. Natural TG fish oil results in 50 % more plasma EPA and DHA after
absorption in comparison to EE oils11, TG forms of EPA and DHA were
shown to be 48 % and 36 % better absorbed than EE forms12, EPA
incorporation into plasma lipids was found to be considerably smaller and took
longer when administered as an EE13, plasma lipid concentrations of
EPA and DHA were significantly higher with daily portions of salmon in
comparison to 3 capsules of EE fish oil14 and in the rat, DHA TG
supplementation led to higher plasma and erythrocyte DHA content than did DHA
EE15 and a higher lymphatic recovery of EPA and DHA16.
One of the causative factors for the poor bioavailability of EE is a much
greater resistance to digestive enzymes. As previously mentioned, during the
digestive process, pancreatic lipase enzymes hydrolyse (cleave) the oils to
liberate the fatty acids and EEs are much more resistant to this enzymatic
process than the natural TG form7. A recent study assessed the
specificity of five lipases towards EPA and DHA in TG and EE forms. All of the
investigated lipases discriminated against both EPA and DHA more in EE than in
the natural TG oils. In other words, both EPA and DHA were more easily
hydrolysed from a TG than from an EE. EPA and DHA hydrolysis would be further
compromised in individuals who suffer from a digestive disorder, such as
pancreatic insufficiency. EEs should be avoided in such populations as they
would likely cause malabsorption of EPA and DHA. Review of the existing
literature provides evidence which, suggests that omega-3 fatty acids in the
natural form of TGs are more efficiently digested and significantly better
incorporated into plasma lipids in comparison to EE forms. Recently, two
clinical trials have settled the debate of which fish oil form is more
bio-available in humans; the ethyl ester (EE) versus the triglyceride (TG)
form. The Dyerberg et al., 2010 study was done to demonstrate the differences
in absorption levels of plasma EPA+DHA following consumption of various fish
oil forms including EE and TG. They noticed that with about the same grand
total of EPA+DHA administered to the EE and TG group compared to the placebo
group, the EE form was given the lowest assimilation as a measure of
bioavailability9. The mechanism of action was simple, in that,
pancreatic lipase breaks down EE to a lesser extent than TG9. Since,
the omega 3 fatty acid plasma profile can significantly be elevated with the
consumption of TG versus EE fish oil; then clearly TG fish oil can be more
effective. In another more recent study done by Neubronner et al., 2010 a
similar comparison was made utilizing a different study design. A unique
method of bio-availability was used (Omega 3 index) this method looks at the
omega 3 FA (EPA+DHA) incorporated into the RBC membranes10. In
comparison to the plasma levels measured in the Dyerberg et al., study, this
method is even more specific because it can measure EPA+DHA at the level of
the tissues10. Therefore, the outcome of this study showed a
statistically significant incorporation of EPA+DHA in the RBC membranes via TG
over EE by over 25 percent10. Therefore, in both of the above
studies the overall bioavailability of omega 3 fatty acids with equal EPA+DHA
in the form of TG showed to be more effective.
Ethyl ester fish oils are less stable, and readily oxidize
Omega-3 fatty acids in the form of EEs are much less stable than those in the
natural TG form and readily oxidize. The oxidation kinetics of DHA as an EE or
as a TG was assessed by measuring the concentration of oxygen found in the
head space of a reaction vessel with both TG and EE forms17. The EE
form of DHA was more reactive, and quickly oxidized, demonstrating that EEs
are far less stable and can more readily produce harmful oxidation products17.
Furthermore, the stability of phospholipid, triglyceride and EEs containing
DHA has been assessed18. After a ten-week oxidation period, the EE
DHA oil decayed 33 % more rapidly18.
Ethyl ester fish oil safety
During the digestive process, EEs are converted back to TGs by intestinal
enterocytes1 which, results in the release of ethanol. Although the amount of
ethanol released in a typical dose of fish oil is small, those with
sensitivities to alcohol or those who are alcoholics should refrain from the
consumption of EEs. Young children may also be more vulnerable to the toxic
effects of ethanol even in small quantities. The exact amount of ethanol
released from the EE fish oil is dependent on the exact profile of the fatty
acids. For a typical 60 % omega-3 EE concentrate the amount of ethanol would
be approximately 15 % by weight (see Figure 1). Additional concern exists
regarding whether a small portion of EE 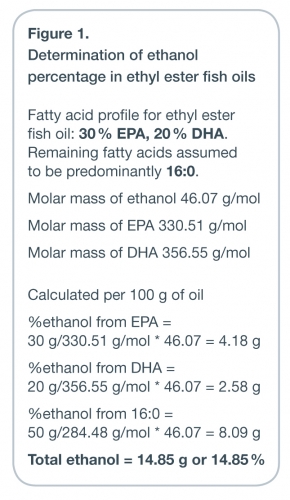 is
absorbed directly into the body19. Unlike TGs, the presence of EEs
in the body has been found to potentiate cytotoxicity19. Several in
vitro studies using purified lysosomes20, purified mitochondria19
or intact Hep G2 cells22 have provided evidence for toxicity of EEs.
Studies in animals have shown that ethanol released into the liver and
pancreas can result in severe organ damage23. Post mortem organ
analysis has demonstrated that EEs are toxic mediators of ethanol induced
cellular injury24, and have been shown to induce pancreatic
injuries when infused in vivo into rats25. It is possible that
efficient EE digestion in the GI tract could prevent toxicity3, but
until further studies carefully examine EE oxidation, the potential for direct
uptake of EEs, or EE absorption into the circulation via the stomach, EEs
should be consumed with caution.
is
absorbed directly into the body19. Unlike TGs, the presence of EEs
in the body has been found to potentiate cytotoxicity19. Several in
vitro studies using purified lysosomes20, purified mitochondria19
or intact Hep G2 cells22 have provided evidence for toxicity of EEs.
Studies in animals have shown that ethanol released into the liver and
pancreas can result in severe organ damage23. Post mortem organ
analysis has demonstrated that EEs are toxic mediators of ethanol induced
cellular injury24, and have been shown to induce pancreatic
injuries when infused in vivo into rats25. It is possible that
efficient EE digestion in the GI tract could prevent toxicity3, but
until further studies carefully examine EE oxidation, the potential for direct
uptake of EEs, or EE absorption into the circulation via the stomach, EEs
should be consumed with caution.
How can I determine if my fish oil is a natural triglyceride or an
ethyl ester?
There is a simple, inexpensive and rapid method to determine if a fish oil
supplement is in the TG or EE form by using polystyrene (Styrofoam) cups.
Method
Measure and place 20 ml of fish oil in a polystyrene cup. Place the cup on a
plate to avoid any mess. Observe the cup after 10 minutes. If the fish oil has
leaked significantly through the cup it contains EE. Due to their chemical
composition, EE will actually eat straight through the polystyrene cup. This
effect will become evident after just a few minutes; however, significant
leakage is seen after 10 minutes. Natural TG fish oils placed in the same cup
will not show leakage after 10 minutes. Natural TG fish oils may show leakage
through the cup in very small amounts after 2-3 hours.
Conclusion
Fish oil supplements in the natural TG form offer numerous advantages when
compared to those in the EE form: oils in a TG form are completely safe to
consume, are naturally occurring, provide increased absorption, and are much
more stable. Therefore, since TG fish oil can be more effectively absorbed
then it can be potentially better at reaching therapeutic ranges in comparison
to EE fish oil. While EEs are a source of omega-3 fatty acids, research shows
that they are not as beneficial as TGs and additional research is required to
fully assess potential toxicity. While some countries (e.g. Australia) have
gone as far as banning the sale of EEs, other countries such as the US,
Canada, and the UK allow the sale of the EE form and furthermore do not
require any additionally labeling. These supplements are therefore often
incorrectly labeled as “Fish Oil” and pose a risk to those who must avoid
ingestion of alcohol.
Table 1
|
Studies
comparing the absorption of triglyceride
and ethyl ester Fish Oils Cod Liver Oil
|
| Hansen
JB, Olsen JO, Wilsgard L, Lyngmo V, Svensson B. Comparative effects of
prolonged intake of highly purified fish oils as ethyl ester or
triglyceride on lipids, haemostasis and platelet function in
normolipaemic men. Eur J Clin Nutr,47, 497-507. |
31
normolipaemic non-obese men (21-47 yrs) were given 4 g highly purified
omega-3 ethyl ester fatty acids, 4 g corn oil as a placebo, or 12 g
n-3 triglycerides for 7 weeks. The daily intake of eicosapentaenoic
acid (EPA) and docosahexaenoic acid (DHA) was 2.2 and 1.4 for TG, and
2.2 and 1.2 for EE. Blood samples were collected at week 1, 3 and 7.
Comparison of time course incorporation of n-3 fatty acids in plasma
phospholipids by repeated measures of variance did not show any
difference between the TG and EE n-3 sources. Repeated measure ANOVA
did however reveal a significant difference between TG and EE with
respect to the incorporation of EPA into plasma cholesterol esters.
Argument is made that higher amounts of omega-3 fatty acid lead to
decreased differences between absorptions. Although higher doses of
omega-3 fatty are not always realistic.
|
Beckermann
B, Beneke M, Seitz I. 1990. Comparative bioavailability of
eicosapentaenoic acid and docosahexaenoic acid from triglycerides,
free fatty acids and ethyl esters in volunteers. Arzneimittelforschung,
40(6):700-4.
|
The
bioavailability of EPA and DHA from triglycerides, free fatty acids
and ethyl esters was investigated in 8 female volunteers in a
randomized triple cross-over trial with baseline control. EPA/DHA was
administered in capsules in form of triglycerides, free fatty acids
and ethyl esters. The resulting EPA/DHA plasma levels were determined
and evaluated. The mean relative bioavailability of EPA/DHA compared
to triglycerides was 186/136 % from free fatty acids and 40/48 % from
ethyl esters. Maximal plasma levels were about 50 % higher with free
fatty acids and about 50 % lower with ethyl esters as compared to
triglycerides. The tolerability of the free fatty acids was much worse
than that of triglycerides and ethyl esters. The main side effect was
eructation.
|
Krokan
HE, Bjerve KS, Mork E. 1993. The enteral bioavailability of
eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl
esters as from glyceryl esters in spite of lower hydrolytic rates by
pancreatic lipase in vitro. Biochim Biophys Acta,1168, 59-67.
|
Enteral
absorption by healthy male volunteers of EPA and DHA from an ethyl
ester andnatural triglyceride fish oil was found to be similar after
intake of equivalent doses, however; hydrolysis of natural
triglyceride fish oil was more efficient. In spite of the similar
serum levels of EPA and DHA obtained in vivo, in vitro hydrolysis by
porcine pancreatic lipase of the ethyl ester was 3-fold slower than
hydrolysis of a the triglyceride. Under similar conditions release of
AA from triglyceride and ethyl ester was essentially similar and
approx. 1.5-fold faster than release of EPA and DHA from ethyl esters.
There are therefore differences in the rate of hydrolysis of ethyl
ester and triglycerides fish oils.
|
el
Boustani S, Colette C, Monnier L, Descomps B, Crastes de Paulet A,
Mendy F. (1987). Enteral absorption in man of eicosapentaenoic acid in
different chemical forms. Lipids, 10, 711-4.
|
After
administering the equivalent of 1 g of EPA in four different chemical
forms, the kinetics of EPA incorporation into plasma triglycerides
were compared by gas liquid chromatography on a capillary column
following separation of the lipid fraction by thin layer
chromatography. EPA incorporation into plasma triglycerides was
markedly smaller and later when EPA was administered as an ethyl ester
rather than as EPA free fatty acid, EPA arginine salt or
1,3-dioctanoyl-2-eicosapentaenoyl glycerol. Our results and the data
in the literature are compatible with the hypothesis that the glycerol
form of EPA is absorbed with minimum hydrolysis and escapes random
distribution between the other positions of the glycerol molecule
during the absorption process.
|
Lawson
LD, Hughes BG. (1988). Human absorption of fish oil fatty acids as
triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res
Commun, 52, 328-35.
|
As
triacylglycerols, eicosapentaenoic acid (1.00 g) and docosahexaenoic
acid (0.67g) were absorbed only 68 % and 57 % as well as the free
acids. The ethyl esters were absorbed only 20 % and 21 % as well as
the free acids. The incomplete absorption of eicosapentaenoic and
docosahexaenoic acids from fish oil triacylglycerols correlates well
with known in vitro pancreatic lipase activity.
|
| Visioli
F, Rise P, Barassi MC, Marangoni F, Galli C. (2003). Dietary intake of
fish vs. formulations leads to higher plasma concentrations of n-3
fatty acids. Lipids, 38, 415-8. |
For
six weeks, volunteers were given 100 g/d of salmon, or 1 or 3 capsules
of ethyl ester fish oil/d. Marked increments in plasma EPA and DHA
concentrations (microgram/mg total lipid) and percentages of total
fatty acids were recorded at the end of treatment with either omega-3
capsules or salmon. Increments in plasma EPA and DHA concentration
after salmon intake were significantly higher than after
administration of capsules. The same increments would be obtained with
at least two and nine-fold higher doses of EPA and DHA, respectively,
if administered with capsules rather than salmon. We provide
experimental evidence that natural omega-3 fatty acids from fish are
more effectively incorporated into plasma lipids than when
administered as capsules.
|
| Valenzuela
A, Valenzuela V, San hueza, J, Nieto S. (2005). Effect of
supplementation with docosahexaenoic acid ethyl ester and sn-2
docosahexaenyl monoacylglyceride on plasma and erythrocyte fatty acids
in rats. Ann Nutr Metab. 49, 49-53. |
Female
rats received a 40 day supplementation of either DHA ethyl ester or
DHAmonoglycerate. Plasma and erythrocyte fatty acid composition were
assessed by gas chromatography at day 0 and 40 of supplementation. DHA
ethyl ester increased plasma and erythrocyte DHA by 15 and 11.9 %,
respectively, with no modification of arachidonic acid (AA) con tent.
DHA-monoglycerate supplementation increased plasma and erythrocyte DHA
by 24 and 23.8 %, respectively, and reduced AA by 5.5 and 3 %,
respectively. Although this data is done with animals, the authors
conclude that in the rat, DHAmonoglycerate supplementation allows a
higher plasma and erythrocyte DHA content than DHA-ethyl ester.
|
| Ikeda
I, Sasaki E, Yasunami H, Nomiyama S, Nakayama M, Sugano M, Imaizumi K,
Yazawa K. (1995). Digestion and lymphatic transport of
eicosapentaenoic and docosahexaenoic acids given in the form of
triacylglycerol, free acid and ethyl ester in rats. Biochim Biophys
Acta; 1259: 297-304. |
Lymphatic
transport of EPA and DHA with trieicosapentaenoyl glycerol (TriEPA)
and tridocosahexaenoyl glycerol (TriDHA) was compared with the
transport of ethyl ester and free acid in rats cannulated with
thoracic duct. Trioleoylglycerol (TO) served as a control. Lymphatic
recovery of EPA and DHA in rats given TriEPA and TriDHA was
significantly higher at the first 3 h after the administration
compared to those given as free acid or ethyl ester. The 24-h recovery
was comparable between triacylglycerol (TAG) and free acid, while it
was significantly lower in ethyl ester. The hydrolysis rate of ethyl
esters was extremely low even in 6 h incubation with lipase. Although
this data is done with animals, the authors conclude that there is
less lymphatic recover of EPA and DHA when they are in ethyl ester
form.
|
| Nordoy
A, Barstad L, Connor WE, Hatcher L. 1991. Absorption of the n-3
eicosapentaenoic and docosahexaenoic acids as ethyl esters and
triglycerides by humans. Am J Clin Nutr. 53:1185-90. |
Five
normolipemic subjects received three test meals. 1) 40g n-3
triglycerides, 2) 28 g n-3 ethyl ester plus 12 g olive oil, 3) 28 g
n-3 ethyl ester and 4) 40g olive oil. When equivalent amounts of fat
were given, the increase in chylomicrons and plasma triglycerides was
similar; n-3 fatty acid contents were also similar after n-3 fatty
acid intake as ethyl esters or triglycerides. Ethyl esters alone were
well absorbed and produced similar n-3 fatty acid responses in plasma
triglycerides and chylomicrons. At 24 h after the n-3 fatty acid
containing meals, the fatty acid plasma concentration of these acids
was similar. This study suggests that n-3 fatty acids given as ethyl
esters or triglycerides were equally well absorbed. However, the doses
of fish oil given were unrealistically high thus one should be
hesitant to draw conclusions from such data.
|
| J
Dyerberg , P Madsen , JM Moller ,I Aardestrup ,EB Schmidt.
Bioavailability of marine n-3 fatty acid formations. Prostaglandins
Leutkot. Essent. Fatty Acids 83 (2010),137-141. |
Seventy-
two volunteers were split into 6 groups 4 of which were double blinded
and 2 of which were the EE and rTG groups. Each group was given
approximately the same amount of fish oil 3.1-3.6 grams and then
compared to a corn oil fed placebo group. Base line plasma cholesterol
esters (CE), phospholipids (PL) and triglycerides (TG) were measured
as the mean increase as a grand total of the EPA+DHA present and then
taken again at the end of the two week period9. They noticed that with
about the same grand total of EPA+DHA administered to the EE and rTG
group compared to the placebo group, the EE form was given the lowest
assimilation as a measure of bioavailability. Once adjusted for the
results were 76% and 134% for the EE and rTG groups respectively.
|
| J
Neubronner , JP Schuchardt, G Kressel, M Merkel, C von Schacky and A
Hahn. Enhanced increase of omega-3 index in response to long term n-3
fatty acid supplementation from triacylglycerides versus ethyl esters.
Eur. J. of Clin. Nutr.(2010),1-8. |
The
study randomized 150 subjects in one of three groups; two fish oil
groups versus placebo. The two fish oil groups (EE and rTAG) had the
exact amount of combined EPA+DHA per capsule and the total dose per
day was 1.68grams. The two fish oil groups were compared to a corn oil
placebo group and the duration of the study was 6 months. A unique
method of bio-availability was used (Omega 3 index) this method looks
at the omega 3 FA (EPA+DHA) incorporated into the RBC membranes.
Therefore, the outcome of this study showed a statistically
significant incorporation of EPA+DHA in the RBC membranes via
re-esterified triacylglycerides (rTAG) over ethyl esters (EE) by more
than a 25 percent.
|
References
1) Carlier
H., Bernard A, Caseli A. (1991). Digestion and absorption of
polyunsaturated fatty acids. Reprod Nutr Dev; 31: 475-500.
2) Segura R. (1988). Preparation of fatty acid methyl esters by direct
transesterification of lipids with aluminum chloride-methanol. J Chromatogr.;441:99-113.
3) Saghir M, Werner J, Laposata M. (1997). Rapid in vivo
hydrolysis of fatty acid ethyl esters, toxic nonoxidative ethanol metabolites.
Am J Physiol.;273:G184-90.
4) Mogelson S, Pieper SJ, Lange LG. (1984). Thermodynamic bases for
fatty acid ethyl ester synthase catalyzed esterification of free fatty acid
with ethanol and accumulation of fatty acid ethyl esters. Biochemistry. 1984
Aug 28;23(18):4082-7.
5) Fave G, Coste TC and Armand M. (2004). Physicochemical properties of
lipids: New strategies to manage fatty acid bioavailability.
Cellular and Molecular BiologyTM 50 (7), 815-831
6) Lambert MS, Botham KM, Mayes PA. (1997). Modification of the fatty
acid composition of dietary oils and fats on incorporation into chylomicrons
and chylomicron remnants. Br J Nutr.;76:435-45
7) Yang LY, Kuksis A, Myher JJ. (1990). Lipolysis of menhaden oil
triacylglycerols and the corresponding fatty acid alkyl esters by pancreatic
lipase in vitro: a reexamination. J Lipid Res. 31(1):137-47.
8) Yang LY, Kukis A, Myher JJ. (1990). Intestinal absorption of
menhaden and rapeseed and their fatty acid methyl and ethyl esters in the rat.
Biochem Cell Biol.;68:480-91
9) J Dyerberg , P Madsen , JM Moller ,I Aardestrup ,EB Schmidt.
Bioavailability of marine n-3 fatty acid formations. Prostaglandins Leutkot.
Essent. Fatty Acids 83 (2010),137-141.
10) J Neubronner , JP Schuchardt, G Kressel, M Merkel, C von Schacky and A
Hahn. Enhanced increase of omega-3 index in response to long term n-3 fatty
acid supplementation from triacylglycerides versus ethyl esters. Eur. J. of
Clin. Nutr.(2010),1-8.
11) Beckermann B, Beneke M, Seitz I. (1990). Comparative bioavailability of
eicosapentaenoic acid and docosahexaenoic acid from triglycerides, free fatty
acids and ethyl esters in volunteers. Arzneimittelforschung; 40(6):700-704.
12) Lawson LD, Hughes BG. (1988). Human absorption of fish oil fatty acids as
triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res Commun, 52,
328-335.
13) el Boustani S, Colette C, Monnier L, Descomps B, Crastes de Paulet A,
Mendy F. (1987). Enteral absorption in man of eicosapentaenoic acid in
different chemical forms. Lipids; 10: 711-714.
14) Visioli F, Rise P, Barassi MC, Marangoni F, Galli C. (2003). Dietary
intake of fish vs. formulations leads to higher plasma concentrations of n-3
fatty acids. Lipids; 38: 415-418.
15) Valenzuela A, Valenzuela V, Sanhueza J, Nieto S. (2005). Effect of
supplementation with docosahexaenoic acid ethyl ester and sn-2 docosahexaenyl
monoacylglyceride on plasma and erythrocyte fatty acids in rats. Ann Nutr
Metab; 49: 49-53.
16) Ikeda I, Sasaki E, Yasunami H, Nomiyama S, Nakayama M, Sugano M, Imaizumi
K, Yazawa K. (1995). Digestion and lymphatic transport of
eicosapentaenoic and docosahexaenoic acids given in the form of
triacylglycerol, free acid and ethyl ester in rats. Biochim Biophys Acta;
1259: 297-304.
17) Yoshii H, Furuta T, Siga H, Moriyama S, Baba T, Maruyama K, Misawa Y, Hata
N, Linko P. (2002). Autoxidation kinetic analysis of docosahexaenoic
acid ethyl ester and docosahexaenoic triglyceride with oxygen sensor.
Biosci Biotechnol Biochem;66:749-753.
18) Song JH, Inoue Y, Miyazawa T. (1997). Oxidative stability of
docosahexaenoic acid-containing oils in the form of phospholipids,
triacylglycerols, and ethyl esters. Biosci Biotechnol Biochem. 61(12):2085-8
19) Best CA, Laposata M. (2003). Fatty acid ethyl esters: toxic
non-oxidative metabolites of ethanol and markers of ethanol intake. Front
Biosci; 8: 202-17.
20) Habber TS., Wilson JS, Minoti VA, Pirola RC. (1991). Fatty
acid ethyl esters increase rat pancreatic lysosomal fragility. J. Lab.
Clin. Med. 121:75-764
21) Lange, L. G., and B. E. Sobel. (1983). Mitochondrial dysfunction
induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J.
CZin. Invest. 72: 724-731,1983.
22) Szczepiorkowski, Z. RI., G. R. Dickersin, and M. Laposata. (1995)Fatty
acid ethyl esters decrease human hepatoblastoma cell proliferation and protein
synthesis. GastroenteroZogy 108: 515- 522.
23) Yuan GJ, Zhou XR, Gong ZJ, Zhang P, Sun XM, Zheng SH. (2006).
Expression and activity of inducible nitric oxide synthase and endothelial
nitric oxide synthase correlate with ethanol-induced liver injury. World
J Gastroenterol,12, 2375-2381.
24) Laposata EA, Lange LG. (1986). Presence of nonoxidative ethanol
metabolism in human organs commonly damaged by ethanol abuse. Science;231:
497–9.
25) Werner J, Laposata M, Fernandez-del Castillo C, Saghir M, Iozzo RV,
Lewandrowski KB, Warshaw AL. (1997). Pancreatic injury in rats induced
by fatty acid ethyl ester, a nonoxidative metabolite of alcohol.
Gastroenterology;113: 286–94.
26) Hansen JB, Olsen JO, Wilsgård L, Lyngmo V, Svensson B. (1993).
Comparative effects of prolonged intake of highly purified fish oils as ethyl
ester or triglyceride on lipids, homeostasis and platelet function in
normolipaemic men. Eur J Clin Nutr;,47: 497-507.
27) Krokan HE, Bjerve KS, Mørk E. (1993). The enteral bioavailability
of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters
as from glyceryl esters in spite of lower hydrolytic rates by pancreatic
lipase in vitro. Biochim Biophys Acta; 1168: 59-67.
28) Harris WS, Zucker ML, Dujovne CA. (1988). Omega-3 fatty acids in
hypertriglyceridemic patients: triglycerides vs methyl esters. Am J Clin
Nutr; 48: 992-997
29) Nordøy A, Barstad L, Connor WE, Hatcher L. (1991). Absorption of
the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and
triglycerides by humans. Am J Clin Nutr 53:1185-90.
Printen

(25-09-2018)
 is
absorbed directly into the body19. Unlike TGs, the presence of EEs
in the body has been found to potentiate cytotoxicity19. Several in
vitro studies using purified lysosomes20, purified mitochondria19
or intact Hep G2 cells22 have provided evidence for toxicity of EEs.
Studies in animals have shown that ethanol released into the liver and
pancreas can result in severe organ damage23. Post mortem organ
analysis has demonstrated that EEs are toxic mediators of ethanol induced
cellular injury24, and have been shown to induce pancreatic
injuries when infused in vivo into rats25. It is possible that
efficient EE digestion in the GI tract could prevent toxicity3, but
until further studies carefully examine EE oxidation, the potential for direct
uptake of EEs, or EE absorption into the circulation via the stomach, EEs
should be consumed with caution.
is
absorbed directly into the body19. Unlike TGs, the presence of EEs
in the body has been found to potentiate cytotoxicity19. Several in
vitro studies using purified lysosomes20, purified mitochondria19
or intact Hep G2 cells22 have provided evidence for toxicity of EEs.
Studies in animals have shown that ethanol released into the liver and
pancreas can result in severe organ damage23. Post mortem organ
analysis has demonstrated that EEs are toxic mediators of ethanol induced
cellular injury24, and have been shown to induce pancreatic
injuries when infused in vivo into rats25. It is possible that
efficient EE digestion in the GI tract could prevent toxicity3, but
until further studies carefully examine EE oxidation, the potential for direct
uptake of EEs, or EE absorption into the circulation via the stomach, EEs
should be consumed with caution.