Kurkuma en de ziekte van Parkinson*
Eerder studies hebben al laten zien dat kurkuma goed kan werken tegen de
ziekte van Parkinson. In deze
laboratoriumstudie werd nader bekeken hoe kurkuma werkt tegen deze ziekte. De twee belangrijkste kenmerken van de ziekte van Parkinson zijn het progressief verlies van de dopaminerge neuronen in de substantia nigra en de aanwezigheid van het eiwit α-synucleïne bevattende intracellulaire inclusies. De hoeveelheid en de structuur van dit eiwit beïnvloedt de progressie van de ziekte duidelijk. Uit de studie blijkt dat
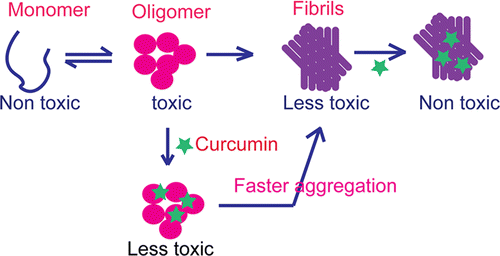
curcumine, de bioactieve stof in kurkuma zich niet rechtstreeks aan het eiwit α-synucleïne bindt doch tot oligomeren en daardoor zorgt voor een vermindering van de toxische structuren van α-synucleïne.
Research Article
Curcumin Modulates α-Synuclein Aggregation and Toxicity
Pradeep K. Singh †‡, Vasudha Kotia †‡, Dhiman Ghosh †‡, Ganesh M. Mohite †‡, Ashutosh Kumar †‡, and Samir K. Maji *†‡
†Department of Biosciences and Bioengineering and ‡Wadhwani Research Centre for Biosciences and Bioengineering, Indian Institute of Technology Bombay, Mumbai, Maharashtra, India 400076
ACS Chem. Neurosci., 2013, 4 (3), pp 393–407
DOI: 10.1021/cn3001203
Copyright © 2012 American Chemical Society
*Department of Biosciences and Bioengineering, IIT Bombay, Powai, Mumbai, India 400076. Telephone: +91-22-2576-7774. Fax: +91-22-2572 3480. E-mail:
samirmaji@iitb.ac.in.
Abstract

In human
beings, Parkinson’s disease (PD) is associated with the oligomerization and amyloid formation of α-synuclein (α-Syn). The polyphenolic Asian food ingredient curcumin has proven to be effective against a wide range of human diseases including cancers and neurological disorders. While curcumin has been shown to significantly reduce cell toxicity of α-Syn
aggregates, its mechanism of action remains unexplored. Here, using a series of biophysical
techniques, we demonstrate that curcumin reduces toxicity by binding to preformed oligomers and fibrils and altering their hydrophobic surface
exposure. Further, our fluorescence and two-dimensional nuclear magnetic resonance (2D-NMR) data indicate that curcumin does not bind to monomeric α-Syn but binds specifically to oligomeric
intermediates. The degree of curcumin binding correlates with the extent of α-Syn
oligomerization, suggesting that the ordered structure of protein is required for effective curcumin binding. The acceleration of aggregation by curcumin may decrease the population of toxic oligomeric intermediates of α-Syn.
Collectively; our results suggest that curcumin and related polyphenolic compounds can be pursued as candidate drug targets for treatment of PD and other neurological
diseases. (Maart 2013)
Reacties:![]()