Two
of the world’s largest drug companies are paying hundreds of millions of
dollars to doctors every year in return for giving their patients anemia
medicines, which regulators now say may be unsafe at commonly used doses.
The
payments are legal, but very few people outside of the doctors who receive
them are aware of their size. Critics, including prominent cancer and kidney
doctors, say the payments give physicians an incentive to prescribe the
medicines at levels that might increase patients’ risks of heart attacks or
strokes.
Industry
analysts estimate that such payments — to cancer doctors and the other big
users of the drugs, kidney dialysis centers — total hundreds of millions of
dollars a year and are an important source of profit for doctors and the
centers. The payments have risen over the last several years, as the makers of
the drugs, Amgen and Johnson & Johnson, compete for market share and try
to expand the overall business.
Neither
Amgen nor Johnson & Johnson has disclosed the total amount of the payments.
But documents given to The New York Times show that at just one practice in
the Pacific Northwest, a group of six cancer doctors received $2.7 million
from Amgen for prescribing $9 million worth of its drugs last year.
Yesterday,
the Food and Drug Administration added to concerns about the drugs, releasing
a report that suggested that their use might need to be curtailed in cancer
patients. The report, prepared by F.D.A. staff scientists, said no evidence
indicated that the medicines either improved quality of life in patients or
extended their survival, while several studies suggested that the drugs can
shorten patients’ lives when used at high doses. Yesterday’s report
followed the F.D.A.’s decision in March to strengthen warnings on the
drugs’ labels.
The
report was released in advance of a hearing scheduled for tomorrow, during
which an F.D.A. advisory panel will consider whether the drugs are overused.
The
medicines — Aranesp and Epogen, from Amgen; and Procrit, from Johnson &
Johnson — are among the world’s top-selling drugs, with combined sales of
$10 billion last year. In this country, they represent the single biggest drug
expense for Medicare and are given to about a million patients each year to
treat anemia caused by kidney disease or cancer chemotherapy.
Dr.
Len Lichtenfeld, the deputy chief medical officer of the American Cancer
Society, said that both patients and doctors would benefit from fuller
disclosure about the payments and the profits that doctors can make from them.
“I suspect that Medicare is going to take a very careful look at what is
going on here,” he said.
Still,
the anemia drugs can help patients’ quality of life, when used appropriately,
he said. “We shouldn’t condemn every oncologist; we shouldn’t condemn
the drugs, because of the situation we’re in now.”
Federal
laws bar drug companies from paying doctors to prescribe medicines that are
given in pill form and purchased by patients from pharmacies. But companies
can rebate part of the price that doctors pay for drugs, like the anemia
medicines, which they dispense in their offices as part of treatment. The
anemia drugs are injected or given intravenously in physicians’ offices or
dialysis centers. Doctors receive the rebates after they buy the drugs from
the companies. But they also receive reimbursement from Medicare or private
insurers for the drugs, often at a markup over the doctors’ purchase price.
Medicare
has changed its payment structure since 2003 to reduce the markup, but private
insurers still often pay more. Combined with those insurance reimbursements,
the rebates enable many doctors to profit substantially on the medicines they
buy and then give to patients.
The
rebates are related to the amount of drugs that doctors buy, and physicians
that agree to use one company’s drugs exclusively typically receive higher
rebates.
Johnson
& Johnson said yesterday in a statement that its rebates were not intended
to induce doctors to use more medicine. Instead, the rebates “reflect
intense competition” in the market for the drugs, the company said.
Amgen
said that rebates were a normal commercial practice and that it had always
properly promoted its drugs.
“Amgen
is dedicated to patient safety,” said David Polk, a spokesman. “We believe
our contracts support appropriate anemia management and our product promotion
is always strictly within the label.”
Both
companies’ stocks fell yesterday after release of the F.D.A. report. Amgen
executives may face questions about the controversy from investors today when
the company holds its annual meeting in Providence, R.I.
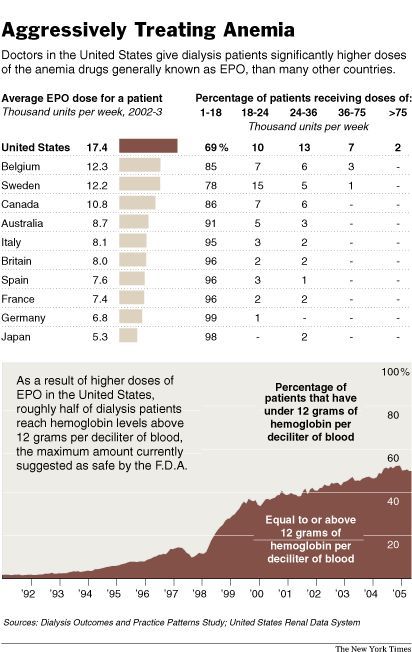
Since
1991, when the first of the drugs was still relatively new, the average dose
given to dialysis patients in this country has nearly tripled. About 50
percent of dialysis patients now receive enough of the drugs to raise their
red blood cell counts above the level considered risky by the F.D.A.
American
patients receive far more of the anemia drugs than patients elsewhere, with
dialysis patients in this country getting doses more than twice as high as
their counterparts in Europe. Cancer care shows a similar pattern. American
cancer patients are about three times as likely as those in Europe to get the
drugs, and they receive somewhat higher doses.
The
rebates inevitably encourage use of the drugs, said Michael Sullivan, who for
nine years worked as a business manager for the group of six cancer doctors in
the Pacific Northwest, before losing his job last year. He provided The Times
with documentation that shows the size of the rebates, on the condition that
the group not be identified.
“Personally,
I think rebates should go away,” said Mr. Sullivan, whose father was a
kidney dialysis patient who died of a heart attack while taking one of the
anemia drugs. “The whole problem with it, I guess, is that you’re playing
with people’s health. It’s not the same as buying widgets.”
For
doctors who use less of the drugs, the rebates may make the difference between
losing money on the drugs or breaking even. Mr. Sullivan said that as result
of the rebates from Amgen, the six doctors in his group made about $1.8
million in net profit on the drugs they prescribed.
Unlike
most drugs, the anemia medicines do not come in fixed doses. Therefore,
doctors have great flexibility to increase dosing — and profits. Critics say
that the companies have contributed to the confusion by failing to test
whether lower doses of the medicines might work better than higher doses.
“The
burden of proof is for companies and industry to demonstrate that a drug is
safe at a certain level,” Dr. Ajay Singh, an associate professor at Harvard
Medical School. Dr. Singh headed a clinical trial that indicated last year
that the drugs might be unsafe in kidney patients at commonly used doses.
Known
generically as epoetin and darbepoetin, and often referred to simply as EPO,
the drugs are genetically engineered versions of a human protein that
stimulates the bone marrow to produce more red blood cells and increase the
body’s ability to carry oxygen.
Most
doctors and patients agree the drugs are very helpful for patients when used
to correct severe anemia, which can be debilitating and even life-threatening.
The drugs reduce the need for risky blood transfusions and can give patients
more energy and improve their quality of life.
“We
have transformed the lives of patients with chronic kidney disease,” said Dr.
Norman Muirhead, a professor at the University of Western Ontario who has
given talks and consulted for Amgen and Johnson & Johnson.
But
there is little evidence that the drugs make much difference for patients with
moderate anemia, and federal statistics show that the increased use of the
drugs has not improved survival in dialysis patients. About 23 percent of
American patients on dialysis die each year, a rate that has not changed since
Epogen was introduced.
Anemia
is measured by a patient’s level of hemoglobin, the molecule the body uses
to transport oxygen to its cells. Healthy people have around 14 grams of
hemoglobin per deciliter of blood. Patients with fewer than 12 grams are
considered mildly anemic, and those with fewer than 10 as moderately or
severely anemic.
The
labels on the drugs, as currently approved by the F.D.A., encourage doctors to
aim for a hemoglobin level of 10 to 12. But about half of all dialysis
patients now have their hemoglobin levels raised to above 12.
Critics
of the drugs say their increased use has been driven by profit. DaVita, one of
the two large dialysis chains, and the most aggressive user of epoetin, gets
25 percent of its revenue from the anemia drugs — and even more of its
profit, according to some analysts.
Dr.
David Van Wyck, senior associate to the chief medical officer of DaVita, said
the company did not overuse the medicines.
Doctors
determine how much to use, Dr. Van Wyck said. “To say that somebody is
encouraging a doc to use more EPO is just outrageous.”
Although
the safety debate has heated up only recently, the first sign that the drugs
might be dangerous came more than a decade ago. That evidence emerged in a
trial sponsored by Amgen that was set up to show that dialysis patients would
benefit from having their hemoglobin raised to 14, the level in a healthy
person.
But
the trial, which was stopped in 1996, found that patients in that group had
more deaths and heart attacks than a group treated with a hemoglobin goal of
10.
That
trial should have discouraged doctors from using too much epoetin and
encouraged Amgen to study the risks further, said Dr. Steven Fishbane, a
nephrologist at Winthrop-University Hospital on Long Island.
Instead,
use of epoetin continued to soar. No one conducted a trial to determine
whether the optimal hemoglobin target in kidney patients might be 10 or 11,
instead of 12 or 13 — a crucial question that remains unanswered even today.
Dr.
Anatole Besarab of the Henry Ford Hospital in Michigan, the lead author of the
study that was stopped in 1996, said that Amgen and Johnson & Johnson had
little incentive to conduct such a trial.
Dr.
Robert M. Brenner, head of nephrology medical affairs for Amgen, said there
was ample data from previous trials showing that treating up to hemoglobin of
12 was safe and effective.
Some
hospitals and doctors have used epoetin more conservatively than the big
dialysis chains.
Dr.
Ronald A. Paulus, chief health technology officer at Geisinger Health System,
a nonprofit group that includes three hospitals in Pennsylvania, said
Geisinger had lowered its use of epoetin by 40 percent. Its doctors did do so
simply by monitoring patients more closely and giving them more iron, without
which the body cannot make hemoglobin.
Dr.
N. D. Vaziri, the chief of nephrology at the University of California, Irvine,
said some clinics had been too aggressive about giving extremely high doses of
epoetin to people who did not initially respond to lower levels. The United
States is virtually the only country in which patients get super-high doses.
“You
create a toxicity situation,” said Dr. Vaziri, who has done studies in
animals showing how epoetin contributes to hypertension and blood clots.
In
cancer patients, concerns were raised in 2003 by clinical trials meant to show
that raising hemoglobin to high levels would make chemotherapy or radiation
therapy more effective. Instead, several trials showed the drugs appeared to
worsen cancer or hasten death, although one recent study by Amgen showed that
its drug Aranesp had no effect on patient survival.
The
conflicting studies are among the issues the F.D.A. advisory committee is
expected to discuss tomorrow. Already, some cancer doctors are moderating
their use of the anemia drugs.
Dr.
Peter Eisenberg, an oncologist in Marin County, Calif., said many doctors had
been induced to use more epoetin by the financial incentives and the belief
that the drug was helpful.
“The
deal was so good,” he said. “The indication was so clear and the downside
was so small that docs just worked it into their practice easily.
“Now
it’s much scarier than that,” he said. “We could really be doing harm.”
(Mei 2007) (Opm. Zo zie je maar waar we naar toe gaan in de Westerse gezondheidszorg. Dan is het toch beter de adviezen op deze site proberen op te volgen. Voor zij die denken dat dit alleen maar Amerikaanse toestanden zijn: eerdere onderzoeken laat zien dat in Nederland jaarlijks 1 miljard euro door de farmaceutische industrie uitgegeven wordt aan "marketing". )
09-05-2007