Home / Nutriënten / ...
Vitamin D Status and Arterial Hypertension: A Systematic Review
Stefan Pilz, MD; Andreas Tomaschitz, MD; Eberhard Ritz, MD; Thomas R. Pieber, MD
CME Released: 08/18/2009;
Abstract and Introduction
Abstract
Vitamin D deficiency is common and is primarily caused by a lack of ultraviolet-B (UVB) radiation from reduced sun exposure, and the consequent limiting of vitamin D production in the skin. The vitamin D endocrine system regulates about 3% of the human genome. Observational data support the concept that vitamin D is involved in the pathogenesis of cardiovascular diseases and arterial hypertension. The antihypertensive properties of vitamin D include renoprotective effects, suppression of the renin-angiotensin-aldosterone system, direct effects on vascular cells, and effects on calcium metabolism, including prevention of secondary hyperparathyroidism. The results of clinical studies largely, but not consistently, favor the hypothesis that vitamin D sufficiency promotes lowering of arterial blood pressure. Randomized, placebo-controlled trials are greatly needed to clarify and definitively prove the effect of vitamin D on blood pressure. In general, the antihypertensive effects of vitamin D seem to be particularly prominent in vitamin-D-deficient patients with elevated blood pressure. Thus, in view of the relatively safe and inexpensive way in which vitamin D can be supplemented, we believe that vitamin D supplementation should be prescribed to patients with hypertension and 25-hydroxyvitamin D levels below target
values.
Introduction
Vitamin D insufficiency affects almost 50% of the population worldwide.[1] This pandemic of hypovitaminosis D can mainly be attributed to lifestyle (for example, reduced outdoor activities) and environmental (for example, air pollution) factors that reduce exposure to sunlight, which is required for ultraviolet-B (UVB)-induced vitamin D production in the skin. Levels of UVB radiation diminish with increasing distance from the earth's equator, during the winter months, and as a result of air pollution. Black people absorb more UVB in the melanin of their skin than do white people and, therefore, require more sun exposure to produce same amounts of vitamin D.[2] Importantly, conditions associated with reduced UVB-induced vitamin D production, such as high latitude, industrialization, and dark skin, have all been associated with increased blood pressure values.[2] The logical hypothesis that high UVB-induced vitamin D production is associated with low blood pressure was confirmed by a small trial of 18 patients with untreated essential hypertension.[3] The researchers found that systolic and diastolic blood pressure values were reduced by 6 mmHg after 6 weeks of UVB irradiation three times per week. uVB irradiation was also associated with a 162% rise in plasma 25-hydroxyvitamin D (25[OH]D) concentrations, but in hypertensive patients who received UVA irradiation, no significant change in 25(OH)D levels or blood pressure occurred.[3]
The high prevalence of vitamin D insufficiency is a particularly important public health issue because hypovitaminosis D is an independent risk factor for total mortality in the general population.[4] A meta-analysis published in 2007 showed that vitamin D supplementation was associated with significantly reduced mortality.[5] Furthermore, vitamin D insufficiency is associated with an increased risk of cardiovascular events, but whether this association reflects a causal relationship remains unclear.[6-8] The effect of vitamin D on blood pressure could be one of the potential mechanisms underlying the link between vitamin D and cardiovascular disease. In this Review, we will summarize the mechanisms that are presumed to underlie the relationship between vitamin D and arterial hypertension, and examine the clinical data for this
association.
Vitamin D Metabolism
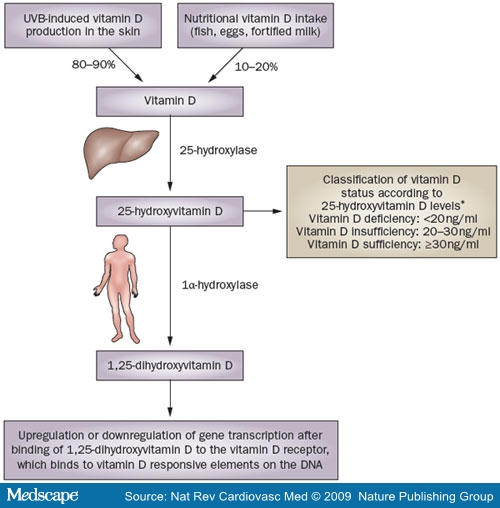
In humans, the primary source of vitamin D is UVB-induced conversion of 7-dehydrocholesterol to vitamin D in the skin.[1] Just 10-20% of our vitamin D comes from dietary sources, such as fish, eggs, or vitamin-D-fortified milk (Figure Vitamin D is hydroxylated in the liver to 25(OH)D—the main circulating vitamin D metabolite, which is largely bound to vitamin D binding protein in serum, and is used to classify vitamin D status: vitamin D sufficient (25[OH]D ≥30 ng/ml [or ≥75 nmol/l]), vitamin D insufficient (25[OH]D 20-30 ng/ml [or 50-75 nmol/l]), and vitamin D deficient (25[OH]D <20 ng/ml [or <50 nmol/l]).[1] These cut-points are currently the most commonly used classification of vitamin D status, but some debate about exact threshold values still exists. Some researchers consider 25(OH)D levels of 10-20 ng/ml (25-50 nmol/l) as vitamin D insufficient and levels below 10 ng/ml (25 nmol/l) as vitamin D deficient, whereas others use a cut-off level of 40 ng/ml (100 nmol/l) to define sufficient vitamin D status.[9,10] 25(OH)D is transformed by renal or extrarenal 1α-hydroxylase into 1,25-dihydroxyvitamin D (1,25[OH]2D), which circulates at much lower serum concentrations than 25(OH)D, but has a much higher affinity to the vitamin D receptor (VDR).[11] Serum levels of 1,25(OH)2D are mainly determined by renal 1,25(OH)2D production, which is closely related to calcium homeostasis, and is upregulated by parathyroid hormone, the concentration of which increases when calcium levels are low.[1,12] In addition, other factors such as fibroblast growth factor 23 and Klotho, which suppress 1α-hydroxylase expression, have also been shown to regulate the renal conversion of 25(OH)D to 1,25(OH)2D.[13] Studies have, however, shown that many other cell types, including those of the vascular wall, express 1α-hydroxylase with subsequent intracellular conversion of 25(OH)D to 1,25(OH)2D, which exerts its effects at the level of the individual cell or tissue before being catabolized to biologically inactive calcitroic acid.[1,12,14] These intracellular tissue levels of 1,25(OH)2D are determined by the concentration of circulating 25(OH)D, which is, therefore considered the best indicator of whole-body vitamin D status. Importantly, extrarenal 1α-hydroxylase expression also underlies various regulatory mechanisms. In this context, extrarenal 1,25(OH)2D production in macrophages is stimulated by Toll-like receptor as part of the innate immune response against intracellular bacteria.[15] Another example of extrarenal regulation of 1α-hydroxylase is the increased production of 1,25(OH)2D by keratinocytes in wounds, which could be induced by transforming growth factor (31.[16] 25(OH)D serum levels, therefore, provide a good estimate of vitamin D status, but regulation of 1α-hydroxylase activity should also be
considered.

Figure 1. Sources and metabolism of vitamin D. In the liver, vitamin D is hydroxylated to 25-hydroxyvitamin D, which is the main circulating vitamin D metabolite and is used for classification of vitamin D status. Further hydroxylation of 25-hydroxyvitamin D to 1,25-dihydroxyvitamin D is catalyzed by 1α-hydroxylase, which is expressed in many different cells throughout the whole body. 1,25-dihydroxyvitamin D binds to the vitamin D receptor, which regulates various genes by binding to vitamin D responsive elements on the DNA. Abbreviation: UVB = ultraviolet-B radiation. *Vitamin D status classification according to reference 1.
1,25(OH)2D binds to the VDR and, after forming a heterodimer with the retinoid X receptor (RXR), binds to specific DNA sequences—the so called 'vitamin D responsive elements'. These sequences are located in the promoter regions of various vitamin-D-dependent genes that are either upregulated or downregulated by the RXR-VDR complex.[1,12,14] Approximately 3% of the human genome is directly or indirectly regulated by the vitamin D endocrine system, which supports the idea that vitamin D insufficiency has widespread adverse consequences for human health.[14] In addition to cardiovascular pathology, vitamin D insufficiency can cause musculoskeletal, malignant, metabolic, or immunological diseases.[1,12,14]
Mechanistic Links With Blood Pressure
The Renin-angiotensin-aldosterone System
Increased activation of the renin-angiotensin-aldosterone system (RAAS), which is a main regulator of electrolyte and volume homeostasis, contributes to the development of arterial hypertension.[17] Renin is mainly synthesized by the juxtaglomerular cells of the kidney and stimulates the production of angiotensin II and of aldosterone, which increase blood pressure directly by vasoconstriction and indirectly by salt and water retention and other mechanisms.[17] Inappropriate, increased activation of the RAAS has been reported in VDR and 1α-hydroxylase knockout mice,[18-21] although it should be acknowledged that the increase in renin activity did not achieve statistical significance in one of these studies.[20] Importantly, VDR and 1α-hydroxylase knockout mice developed arterial hypertension and myocardial hypertrophy, which were present even after normalization of calcium homeostasis; however, blocking of the RAAS system with angiotensin-converting-enzyme inhibitors normalized blood pressure and cardiac abnormali-ties.[18-21] Furthermore, increased RAAS activation, arterial hypertension, and myocardial abnormalities could be successfully treated with 1,25(OH)2D in 1α-hydroxylase knockout mice.[21] The molecular effects of vitamin D on the RAAS have been clarified by the finding that liganded VDR suppresses renin expression by binding to the transcription factor cAMP-response element-binding protein (CREB).[22] As a result, stimulation of renin transcription is inhibited because CREB is no longer able to stimulate renin transcription by binding to cAMP response elements in the promoter region of the renin gene.[22] In patients with arterial hypertension, renin activity has been inversely associated with 1,25(OH)2D levels.[23,24] Importantly, decreased renin and angiotensin II levels were observed in several, but not all, studies that examined the activity of the RAAS after treatment with vitamin D, 1,25(OH)2D or active vitamin D analogs.[25-30] Thus, further studies are needed to clarify the clinical importance of vitamin-D-induced suppression of the RAAS.
Vitamin D, Calcium, and Parathyroid Hormone
Evidence exists that secondary hyperparathyroidism and low serum calcium levels, which are commonly seen in patients with vitamin D deficiency, could partially mediate the link between poor vitamin D status and arterial hypertension. Vitamin D is important for calcium homeostasis: firstly because it increases the synthesis of calcium transport proteins, which are required for active intestinal calcium absorption; secondly, it stimulates calcium reabsorption in the distal renal tubule; and thirdly, it causes release of calcium from bone by stimulating osteoclasts.[1,12] Patients with vitamin D deficiency are, therefore, prone to developing low plasma levels of ionized calcium. These low levels of ionized calcium stimulate the calcium sensing receptor of the parathyroid gland, inducing the release of parathyroid hormone in order to maintain normal calcium levels in states of vitamin D deficiency.[1,12] Parathyroid hormone increases calcium levels primarily by increasing renal 1α-hydroxylase activity, with subsequent conversion of 25(OH)D to 1,25(OH)2D, and by stimulating calcium release from bone and intestinal calcium absorption.[1,12]
In addition to these well known effects on calcium homeostasis, parathyroid hormone has multiple effects on the cardiovascular system.[31] Observational studies have shown that parathyroid hormone is associated with arterial hypertension.[31-33] Furthermore, Hulter et al. demonstrated that intravenous infusion of parathyroid hormone increased blood pressure in apparently healthy individuals.[33] The underlying mechanisms for the association between parathyroid hormone and blood pressure remain unclear, but accumulating evidence indicates that parathyroid hormone affects the vascular smooth muscle cells, which might increase vascular stiffness and promote atherosclerotic changes, particularly in patients with kidney disease.[31] Calcium deficiency, in itself, can also contribute to arterial hyper-tension.[12,34] Notably, however, calcium supplementation seems to have only minimal effects on blood pressure; hypothetically, these effects are mediated by reducing vascular tone or increasing natriuresis.[12,34,35] A number of studies have demonstrated alterations in vitamin D metabolism, such as increased urinary loss of protein bound 25(OH)D, in patients with salt-sensitive hypertension and proteinuria.[36-42] In addition, high sodium load was shown to increase urinary calcium loss and could, therefore, have an important impact on calcium homeostasis, hypothetically by modulating hydroxyla-tion and effects of vitamin D metabolites.[36-42] Further in-depth studies will be required to confirm or deny these
speculations.
Effects on Cells of the Vessel Wall
Vitamin D and its analogs elicit various effects on the cells of the vessel wall, which include endothelial cells, vascular smooth muscle cells, and macrophages, all of which express the VDR as well as 1α-hydroxylase.[1,12,14] In spontaneously hypertensive rats, 1,25(OH)2D reduced endothelium-dependent contractions of the aorta by decreasing cytosolic-free calcium concentrations in endothelial cells.[43] 1,25(OH)2D has also been shown to protect endothelial cells against the harmful effects of advanced glycation end products, which are elevated in patients with diabetes or uremia.[44] 1,25(OH)2D exerts its vasculoprotective effects by decreasing endothelial adhesion molecules, by increasing the activity of endothelial nitric oxide synthase, and through its anti-inflammatory properties.[44,45] In line with these findings, vitamin D deficiency is associated with endothelial dysfunction, and vitamin D supplementation has been shown to improve endothelial function in patients with type 2 diabetes.[25,46]
The effects of vitamin D on vascular smooth muscle cells are complex and are modulated by other hormones, such as parathyroid hormone and estrogenic compounds, which upregulate 1α-hydroxylase in these cells.[47,48] A 1,25(OH)2D-mediated increase in prostacyclin production by vascular smooth muscle cells has been reported, supporting the concept that vitamin D has vasodilatatory and antiatherosclerotic properties.[49,50] Other studies, however, indicate that 1,25(OH)2D could increase vascular resistance by increasing sensitivity to vasoconstrictors.[51-57] In addition, 1,25(OH)2D modulates growth, differentiation, and migration of vascular smooth muscle cells; however, directionally different effects of vitamin D on vascular cells have been reported (possibly the result of various cell culture conditions), and firm conclusions cannot be made.[58-60]
Other Mechanisms
Accumulating molecular and clinical data indicate that vitamin D deficiency contributes to insulin resistance,[61] which is thought to have a role in the pathogenesis of arterial hypertension. Furthermore, renoprotective effects of vitamin D, such as decreasing podocyte loss and podocyte hypertrophy, or suppression of mesangial cell proliferation, might antagonize the development of arterial hypertension in the course of renal failure.[62,63] The potential mechanisms of the antihypertensive effects of vitamin D are summarized in Figure 2.
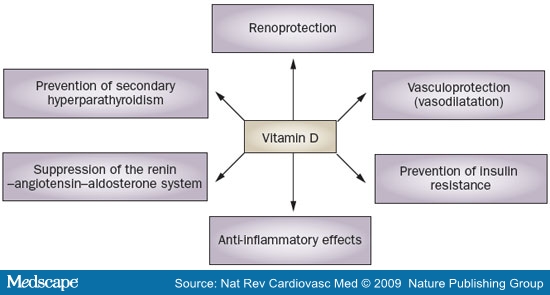
Figure 2. The antihypertensive effects of vitamin D.
Vitamin D and BP: Clinical Data
The association between 25(OH)D levels and arterial hypertension has been assessed in several cross-sectional studies (Table 1).[32,64-88] Notably, blood pressure evaluation in these studies was almost always based on a single measurement (usually taken in the physician's office), which can only give a crude estimate of the average blood pressure level and is susceptible to artifacts (for example, regression to the mean).[89,90] Ambulatory blood pressure monitoring (ABPM)—which is considered by many clinicians to be the 'gold standard' of blood pressure measurement—provides better information about average blood pressure levels in daily life, correlates better with hypertension-induced end-organ damage than office blood pressure levels, and can be used to obtain additional information such as diurnal blood pressure variations.[89,90] However, ABPM and other useful estimates of average blood pressure, such as repeated measurements of office or home blood pressure, have not been used in large clinical studies on vitamin D status and blood
pressure.[89,90]
Table 1. Findings From Cross-sectional Studies of 25(OH)D and Blood Pressure
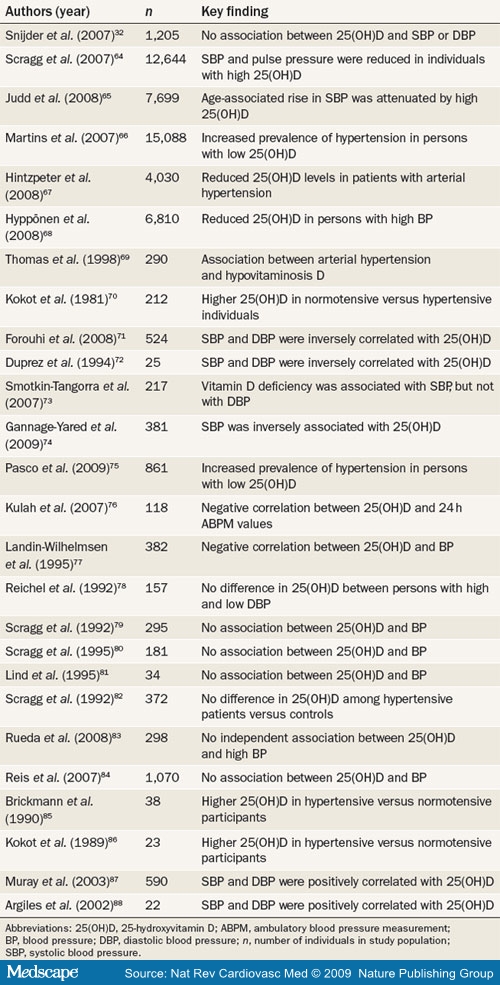
The third National Health and Nutrition Examination Survey (NHANES-III),[64] which is representative of the noninstitutionalized US civilian population, showed that systolic blood pressure and pulse pressure were inversely and significantly correlated with 25(OH)D levels among 12,644 participants. These results were confirmed by subgroup analyses, in which the age-associated increases in systolic blood pressure were significantly lower in individuals with vitamin D sufficiency.[65,66] The prevalence of arterial hypertension was also associated with reduced serum 25(OH)D levels in 4,030 participants of the German National Interview and Examination Survey,[67] in 6,810 participants of the 1958 British Birth Cohort,[68] and in other study populations.[69-77] In other investigations, a significant association between 25(OH)D and blood pressure has not been reported.[78-84] With the exception of the Longitudinal Aging Study Amsterdam[32] (1,205 participants aged 65 years and older) and the population-based Ranch Bernardo Study[84] (410 men and 660 women), most of these studies[78-83] had substantially smaller sample sizes than NHANES-III,[64] the German National Interview and Examination Survey,[67] and the 1958 British Birth Cohort.[68] Interestingly, however, the investigators of the Longitudinal Aging Study Amsterdam[32] and the Ranch Bernardo Study[84] cautioned that the lack of a significant association between vitamin D status and blood pressure might be attributable to the relatively high baseline levels of 25(OH)D among participants in these studies. By contrast, a few studies have reported increased 25(OH)D levels in patients with high blood pressure when compared with normotensive individuals.[85-88] Despite the inconsistent findings from some cross-sectional investigations, the majority of studies with large sample sizes have demonstrated an inverse relationship between 25(OH)D levels and blood pressure.
Only a few prospective studies have addressed the question of whether there is an association between 25(OH)D and change in blood pressure or new-onset hypertension. In the population-based Ely study,[71] no significant correlation was noted between baseline 25(OH)D levels and changes in blood pressure during the 10 years of follow-up, even though there was a significant, inverse association between 25(OH)D levels and systolic and diastolic blood pressures at baseline. Importantly, among 613 men in the Health Professionals' Follow-Up Study (HPFS) and 1,198 women in the Nurses' Health Study (NHS), the risk of incident hypertension was 3.18 (95% CI 1.39-7.29) for individuals with 25(OH)D levels below 15 ng/ml (37.4 nmol/l) compared with those whose 25(OH)D levels were 30 ng/ml (75 nmol/l) or higher.[91] Unfortunately, 25(OH)D concentration was measured in only a minority of the participants in the HPFS and the NHS and so, as a surrogate, the investigators used clinical data—such as sun exposure or nutritional vitamin D intake—to predict 25(OH)D levels for the entire study populations. These 'predicted' 25(OH)D concentrations were available for 38,388 men in the HPFS (9,029 new cases of hypertension over 16 years) and in 77,531 women in the NHS (26,525 new cases of hypertension over 18 years).[91] The multivariable adjusted risk of incident hypertension was 2.31 (95% CI 2.03-2.63) for men of the HPFS and 1.57 (95% CI 1.44-1.72) for women of the NHS, when the lowest decile of the predicted 25(OH)D levels were compared with the highest decile.[91] In addition, a prospective study with a nested case-control design, which included 1,484 healthy women from the second NHS, was also performed.[92] After adjustments for possible confounders, the risk of incident hypertension was 1.66 (95% CI 1.11-2.48), comparing the lowest with the highest quartile of 25(OH)D. A decrease in 25(OH)D levels of 5 ng/ml (12.5 nmol/l) was associated with an adjusted odds ratio for incident hypertension of 1.08 (95% CI 1.01-1.15).[92] A causal role for vitamin D in the pathogenesis of arterial hypertension is also indicated by reports that VDR polymorphisms are associated with increased blood pressure.[87,93] Low levels of 25(OH)D have also been associated with an increased risk of pre-eclampsia[94] and with reduced rates of hypertension resolution after gastric bypass[95] in individuals with morbid obesity.
Studies that have addressed the association between vitamin D intake and blood pressure have found that either there is no significant correlation,[96,97] or that low vitamin D intake is associated with increased blood pres-sure.[34,98,99] As stated earlier, however, nutrition has only a minor influence on vitamin D status and, therefore, the conclusions that can be drawn from these studies are limited.
The results from studies of the association between blood pressure and circulating 1,25(OH)2D concentrations have been inconsistent, with either negative,[81,100] positive,[23,85,86,101] or no independent correlations[78,88,102-104] reported. These findings should be interpreted with caution because 1,25(OH)2D concentrations are primarily related to calcium homeostasis, are confounded by renal function, and do not appropriately reflect whole-body vitamin D status. However, it should also be noted that low 1,25(OH)2D levels are associated with poor outcome in patients with heart failure or chronic kidney disease; the problem with interpreting 1,25(OH)2D serum levels is that patients with vitamin D deficiency or vitamin D insufficiency can have either low, normal, or elevated 1,25(OH)2D
concentrations.[105,106]
The Effect of Supplementation on BP
Vitamin D
To our knowledge, there is only one adequately performed, published trial that was primarily designed to evaluate the impact of vitamin D supplementation on blood pressure.[107] In this double-blind, randomized, controlled trial, Pfeiffer et al. enrolled 148 elderly women (aged 70 years or older) with vitamin D deficiency (25[OH]D levels <25 ng/ml [or <62.4 nmol/l)), who were assigned to receive either 1,200 mg of calcium or 1,200 mg of calcium plus 800 IU of vitamin D daily.[107] After 8 weeks of treatment, both groups showed significant reductions in systolic and diastolic blood pressure values. The reduction in systolic blood pressure was 7.4 mmHg greater in the vitamin D plus calcium group than in the calcium alone group (P = 0.02), but there was no significant difference in diastolic blood pressure between the two groups. A greater proportion of individuals in the vitamin D plus calcium group than in the calcium-only group had reductions in systolic blood pressure of at least 5 mmHg (83% versus 47%).[107] The findings of this trial are supported by data from Krause et al., who showed that UVB-induced increases in 25(OH)D levels are associated with reduced blood pressure.[3]
Various other trials, which were not primarily or adequately designed to evaluate the antihypertensive effects of vitamin D, included measurements of blood pressure before and after vitamin D supplementation.[25,108-116] In most of these trials, participants had 25(OH)D levels within the normal range and were largely free from arterial hypertension at baseline. In addition, blood pressure was generally measured in the office, and more sophisticated blood pressure measurement techniques such as ABPM, were not used. In retrospect, therefore, it is not surprising that individual variations in blood pressure (or changes in antihypertensive treatment during the study) obscured the potential effects of vitamin D intake. One study that stands out included (in contrast to most other trials in this field) a cohort of vitamin-D-deficient patients with a high prevalence of arterial hypertension.[25] This trial, which demonstrated a significant antihypertensive effect of vitamin D, included 34 vitamin-D-deficient patients with type 2 diabetes who were randomly assigned to receive a single dose of 100,000 IU vitamin D or placebo.[25] After 8 weeks of follow-up, the mean office systolic blood pressure was 14 mmHg lower in the vitamin D group compared with the placebo group (P = 0.001). After vitamin D treatment, mean diastolic blood pressure was 5 mmHg lower in the intervention versus the placebo group, but this result was not statistically significant.[25]
In the largest trial in this field—the Women's Health Initiative (WHI)—36,282 postmenopausal women were randomly assigned to receive either 400 IU vitamin D plus 1,000 mg calcium daily or placebo.[112] During follow-up (mean 7 years), changes in systolic and diastolic blood pressure, as well as the frequency of incident hypertension, were not significantly different between the intervention and placebo groups. This null finding was confirmed in various subgroup analyses. One potential explanation for this negative result is that the dose of vitamin D was too low to sufficiently increase the 25(OH)D concentrations to levels that have been documented to significantly improve health outcomes. This theory is also relevant for other vitamin-D-related health outcomes; a 2009 meta-analysis showed that vitamin D intake had no significant effect on the incidence of bone fractures in trials where participants were assigned to a daily intake of 400 IU vitamin D, whereas the risk of fracture was significantly reduced in those who received vitamin D supplementation in studies using doses higher than 400 IU per day.[117] In addition, adherence to treatment in the WHI—defined as use of 80% or more of the assigned study medication—was only 60-63% in the first 3 years of follow-up, and 59% at the end of the trial.[118]
1,25(0H)2D or Active Vitamin D Analogs
A small study in seven patients undergoing hemodialysis showed that a single intravenous dose of 1,25(OH)2D (2 |μg/m2) significantly decreased systolic (-17 mmHg) and diastolic (-8 mmHg) blood pressure when measured 2 h after adminsitration.[119] By contrast, intravenous injection of 1,25(OH)2D (0.02 |μg/kg body weight) in nine patients with essential hypertension and ten healthy male volunteers resulted in no significant change in blood pressure after 2 h. Nevertheless, a transient rise in mean blood pressure was seen after 90-100 min in the patients with hypertension compared with the healthy participants.[120] The difference in baseline 1,25(OH)2D concentration between the hemodialyzed and nonuremic individuals could conceivably account for the different outcomes in these two studies. Small studies with treatment periods of between 1 and 15 weeks have also failed to show significant effects of 1,25(OH)2D administration on blood pressure.[26,121,122] In a study by Alborzi et al., paricalcitol—an active vitamin D analog—was shown to have no significant effect on 24 h ambulatory systolic or diastolic blood pressure in patients with chronic kidney disease, but did reduce albuminuria, which underlines the renoprotective effects of vitamin D treatment.[123] Lind et al. have performed several double-blind, placebo-controlled trials to evaluate the long-term effects of the active vitamin D analog alphacalcidol (1α-hydroxyvitamin D) on blood pressure.[27,124-128] These researchers showed, although not consistently, that alphacalcidol reduced blood pressure (particularly diastolic blood pressure) in various different study
cohorts.[27,124-128]
Vitamin D Toxicity
When discussing the beneficial effects of vitamin D on blood pressure, one must consider that pharmacological doses of vitamin D have been shown to cause arterial hypertension, vascular stiffness, and atherosclerosis in rodents; whether this finding has any relevance for humans is unclear.[129] In humans, vitamin D toxicity and associated hypercalcemia—which can cause reversible hypertension—is observed when 25(OH)D levels are higher than 150 ng/ml (374.4 nmol/l).[1] In clinical trials, vitamin D toxicity was not observed with doses of up to 10,000 IU vitamin D per day, which is approximately the level of vitamin D production that can be achieved by endogenous UVB-induced vitamin D synthesis in the skin.[130,131] Consequently, at 10,000 IU vitamin D per day, and in the absence of increased vitamin D sensitivity (for example, sarcoidosis or tuberculosis), vitamin D supplementation is safe. Presumably there is a wide margin between the level of 25(OH)D needed for vitamin D sufficiency (≥30 ng/ml [or 75 nmol/l]) and the level of toxicity (>150 ng/ml [or >374.4
nmol/l]).
Vitamin D Supplementation
An intake of 1,000 IU (25 µg) of vitamin D per day can be generally assumed to result in an increase in 25(OH)D levels of approximately 10 ng/ml (25 nmol/l).[132,133] Evidence indicates that daily, weekly, and monthly vitamin D dosing frequencies can equally increase serum 25(OH)D levels, which have a half-life of about 1 month. In this context, an oral vitamin D intake of 1,500 IU daily, 10,500 IU once weekly, or 45,000 IU once every 28 days has been demonstrated to result in similar increases of 15-16 ng/ml (37.4-40.0 nmol/l) in 25(OH)D levels.[134] The dose to correct vitamin D deficiency should be sufficiently high to achieve 25(OH)D levels of at least 30 ng/ml (75 nmol/l). For example, a patient with 25(OH)D levels of 10 ng/ml (25 nmol/l) should receive at least 2,000 IU daily, which corresponds to weekly doses of at least 14,000 IU or monthly doses of at least 56,000 IU. Several authors recommend loading doses in the initial phase of treatment (that is, 50,000 IU weekly for 8 weeks or 50,000 IU daily for 1 week) before starting maintenance therapy (that is, at least 1,000 IU vitamin D for a person with initial 25[OH]D levels of 20 ng/ml [50 nmol/l]).[1,10] Individual response to vitamin D doses does, however, vary widely and certain patients, such as those who are obese or suffer from malabsorption, might require much higher vitamin D doses than individuals without comorbidities.[1,133] Measurements of 25(OH)D levels are, therefore, useful to monitor 25(OH)D levels and to allow for adequate correction of the vitamin D dose. 25(OH)D levels should be reassessed 3-6 months after initiation of vitamin D supplementation. In patients with increased vitamin D sensitivity, such as those with sarcoidosis or tuberculosis, calcium should be measured in the initial phase of treatment. One problem with vitamin D treatment is that, although maintaining 25(OH)D levels above 30 ng/ml (75 nmol/l) is generally recommended, no consensus exists about optimal 25(OH)D levels. At present, many researchers recommend maintaining 25(OH)D levels between 30 and 60 ng/ml (75.0-149.8 nmol/l).[1,10] We do not know whether higher levels than this are beneficial or detrimental. Data from NHANES-III indicate a 'J-shaped' association between 25(OH)D levels and mortality, with the highest mortality in persons with the lowest 25(OH)D levels, but with slightly increasing mortality in those with supraphysiological 25(OH)D levels. However, other data indicate that particularly high levels of vitamin D are optimal for cancer
prevention.[4,10]
Conclusions
Accumulating evidence, ranging from insights into molecular mechanisms to the outcome of randomized controlled trials, favors the hypothesis that vitamin D deficiency contributes to arterial hypertension. The antihypertensive effects of vitamin D are mediated by renoprotective effects, suppression of the RAAS, by beneficial effects on calcium homeostasis, including the prevention of secondary hyperparathyroidism, and by vasculoprotection. However, definitive evidence from appropriately powered, controlled, intervention trials is lacking. Some inconsistent results from studies of the relationship between vitamin D status and arterial hypertension have been reported, possibly because the effects of 25(OH)D on blood pressure are not apparent in normotensive individuals with 25(OH)D levels within the normal range. In general, evidence for the antihypertensive effects of vitamin D is strongest in patients with elevated blood pressure and vitamin D deficiency; these patients would, in our opinion, benefit from vitamin D supplementation. In addition to cardiovascular sequelae, vitamin D deficiency has been associated with autoimmune, malignant, neurological, metabolic, and infectious diseases, as well as with bone fractures.[1,12,14,117,130,131] In view of the multiple health benefits of vitamin D and the high prevalence of vitamin D deficiency, as well as the easy, safe, and inexpensive ways in which vitamin D can be supplemented, we believe that the implementation of public health strategies for maintaining a sufficient vitamin D status of the general population is warranted.[1,12,117,130,131]
Sidebar:
Key Points
References
(September
2009)